EXTRACT-public
Thank you for your interest in EXTRACT, a cell extraction routine with native GPU implementation. To receive occasional updates about new releases, to ask questions about EXTRACT usage, or schedule a tutorial session for your lab, please send an email to extractneurons@gmail.com along with your name and institution. (Please be sure to add this email to your contact list so that replies and announcements do not go to your spam folder). Thank you!
The EXTRACT team
EXTRACT is a tractable and robust automated cell extraction tool for calcium imaging, which extracts the activities of cells as time series from both one-photon and two-photon Ca2+ imaging movies. EXTRACT makes minimal assumptions about the data, which is the main reason behind its high robustness and superior performance.
We show an example output of EXTRACT on a low SNR movie, in the figure on the right donated by Dr. Peng Yuan. Please note that this is the raw output, with no post-processing and/or manual annotation/selection by users. This run is a result of a batch processing of >30 sessions, optimized only once at the beginning of the study, with no extra parameter tweaking particular to this session. EXTRACT needs to be optimized per surgery/imaging modality type (practically once in the life-time of a study). For a trained person (feel free to schedule a tutorial for your lab!), this process usually takes around few minutes.
EXTRACT can be thought of as being part of a signal extraction pipeline, as illustrated by the figure on the right. The pipeline takes a raw 1p/2p Ca2+ imaging movie as an input. Then, one performs motion correction and various pre-processing steps. Later, the processed movie is used by the EXTRACT algorithm to perform tractable and robust cell extraction, which puts out the temporal traces and spatial maps for future data analysis. EXTRACT code in this repository provides most of the pipeline (colored as green in the figure). We also provide some links to the external repositories whenever needed (colored as orange in the figure right).
Its main features and the mathematical foundations behind EXTRACT as well as a comparison with state-of-the-art methods can be found in the papers cited below (Links to papers: Inan et al., 2021 and Inan et al., 2017). If you use EXTRACT for your own research, please consider citing these works.
@article{inan2021fast,
author = {Inan, Hakan and Schmuckermair, Claudia and Tasci, Tugce and Ahanonu,
Biafra and Hernandez, Oscar and Lecoq, Jerome and Dinc, Fatih and Wagner,
Mark J and Erdogdu, Murat and Schnitzer, Mark J},
title = {Fast and statistically robust cell extraction from
large-scale neural calcium imaging datasets},
elocation-id = {2021.03.24.436279},
year = {2021},
doi = {10.1101/2021.03.24.436279},
URL = {https://www.biorxiv.org/content/early/2021/03/25/2021.03.24.436279},
eprint = {https://www.biorxiv.org/content/early/2021/03/25/2021.03.24.436279.full.pdf},
journal = {bioRxiv}
}
@article{inan2017robust,
title={Robust estimation of neural signals in calcium imaging},
author={Inan, Hakan and Erdogdu, Murat A and Schnitzer, Mark},
journal={Advances in neural information processing systems},
volume={30},
pages={2901--2910},
year={2017}
}- Installation
- A quick start for beginners
- Advanced aspects
- Questions and comments
- License
- Planned future releases
- Frequently asked questions
It is fairly straightforward to install EXTRACT. Simply download all the files from the repository and include them in the MATLAB path. Installation is complete. EXTRACT makes use of various packages and toolboxes, check out the Dependencies section. An example case of running EXTRACT after installation is provided in Example movie extraction.
See our quick tutorial scripts:
| Nickname | View | Run |
|---|---|---|
| "Newcomer" | 👀 | ▶️ |
EXTRACT algorithm has two inputs, movie and configurations (a struct), and a single output, a struct that contains the information on the extracted cell signals. In its essence, the whole extract code can be run by a single line:
output = extractor(M,config)
In this quick start, we first describe the inputs, specifically the most important objects in the configuration struct (e.g. config). Then, we discuss the components of the output file that contain the single cell activities. Finally, we provide a code for initializing the configurations and performing extraction on an example movie.
M: either an existing 3-D movie matrix in the workspace, or a string/cell that specifies an HDF5 path to a 3-D movie matrix.
If M is a string, it must be in the following format: filepath:dataset (for example: 'example.h5:/data'), but with this shortcut, the dataset name cannot include the character :. Otherwise, for a general case, M can be inputted as a cell such that M{1} = filepath and M{2} = dataset. (for example: M{1} = 'example.h5' and M{2}='/data') In this option, there is no restriction on the filepath or the dataset names.
config: a struct whose fields define the various parameters used for signal extraction (See Configurations for details on config). For a beginner, the most relevant fields are:
-
avg_cell_radius: Radius estimate for an average cell in the movie. It does not have to be precise; however, setting it to a significantly larger or lower value will impair performance. It needs to be set at the start for any movie. A recommended way to set this is to consider the maximum projections of the video across time and pick the radius there (see Example movie extraction). -
num_partitions_x/num_partitions_y: User specified number of movie partitions in x and y dimensions. Running EXTRACT on the whole movie at once could be computationally too expensive or simply impossible. In this case, we divide the input movie into smaller parts. Heuristics suggest that the size of the smaller FOV should not be smaller than 128 pixels in any of the x/y dimensions. -
cellfind_min_snr: Minimum peak SNR (defined as peak value/noise std) value for an object to be considered as a cell. Increase this if you want to decrease the ratio of false-positives at the expense of losing some low SNR cells in the process. Default:1. -
use_gpu: This needs to be 1 to run EXTRACT on GPU, 0 to run EXTRACT on CPU. It is preferably, time-wise, to run EXTRACT on GPU. Default:1. -
trace_output_option: Choose 'raw' for raw traces, 'nonneg' for non-negative traces, 'baseline_adjusted' for non-negative traces adjusted for spatially varying baseline. Check Frequently Asked Questions before using the option 'raw'. Default:baseline_adjusted.
EXTRACT has a helper function that initializes the config struct to the most common configurations:
config=[];
config = get_defaults(config);We will provide the complete example code below in Example movie extraction section.
The output is a struct with four fields: spatial_weights, temporal_weights, info and config. Config includes the configurations used by the algorithm while info provides some algorithm statistics that can later become useful for an expert. For a quick start, two fields are of particular importance:
-
spatial_weights: has the shape [movie_height x movie_width x number_of_cells_found] and is an array of inferred spatial images of the cells found. Spatial weights are particularly important for cell checking (See Cell checking), a process where the user individually inspects the cell candidates and determines whether they are actual cells by looking at the activity in the raw movie. -
temporal_weights: has the shape [number_of_movie_frames x number_of_cells_found] and is an array of inferred calcium traces belonging to each cell. Usually, after some pre-processing that includes cell checking, this is the actual output of EXTRACT that gets used for the remaining of the data analysis pipeline.
Having described how to run the algorithm and interpret the output, we know provide a basic code that can get a first time user started quickly.
%First, load the movie.
load('example.mat');
% By considering the maximum projections, pick an estimate cell radius
figure, imshow(max(M,[],3),[]);
%Initialize config
config=[];
config = get_defaults(config);
%Set some important settings
config.use_gpu=1;
config.avg_cell_radius=7;
config.trace_output_option='nonneg';
config.num_partitions_x=1;
config.num_partitions_y=1;
config.cellfind_min_snr=1;
%Perform the extraction
output=extractor(M,config);
% output=extractor('example.h5:/data',config); % If movie is large, do not pre-load. Use this!
% Perform post-processing such as cell checking and further data analysis.
% Check example_tutorial.m for more in depth tutorial!We have included the file example.mat and example_tutorial.m in this repository to help the reader get started with the basics of EXTRACT.
Here, we discuss some of the more advanced aspects of EXTRACT. We suggest that the user first becomes familiar with the example extraction process before moving forward to the more advanced settings.
In this repository, we are introducing an important part of the cell extraction pipeline for Ca2+ imaging movies. The pipeline includes a motion correction step, for which one can use already existing motion correction algorithms (See for example: NoRMCorre). On the other hand, EXTRACT has a configuration to perform the usual pre-processing steps including taking dF/F, highpass filtering for suppressing excessive background, and circular masking for endoscopic movies (See Configurations).
Check tutorial 3 for the list of all relevant hyperparameters and learn about them. We strongly suggest the regular users to at least give a read to the tutorial 3, as it explains the full range of EXTRACT's capabilities in a concise way.
Here is a list of more advanced configurations:
-
preprocess: EXTRACT does preprocessing steps such as taking dF/F, highpass filtering for suppressing excessive background, and circular masking for endoscopic movies. Set tofalseto skip all preprocessing. Default:true. -
downsample_time_by,downsample_space_by: Downsampling factors. Set to'auto'for automatic downsampling factors based on avg cell radius and avg calcium event time constant. Defaults:1&1. -
multi_gpu:Boolean flag for parralel processing of different movie partitions on multiple GPUs (if applicable) in the GPU mode. Default:false. -
parallel_cpu:Boolean flag for parallel processing of different movie partitions in the CPU mode. This flag is only effective whenuse_gpu = 0. Default:false. -
num_parallel_cpu_workers:Whenconfig.parallel_cpu = 1, this parameter can be used to set the desired number of CPU workers. Default is # of available cores to Matlab - 1 (minus 1 is for leaving compute room for other tasks). -
min_radius_after_downsampling: Whendownsample_space_by= 'auto', this determines the spatial downsampling factor by setting a minimum avg radius after downsampling. Default:5. -
min_tau_after_downsampling: Whendownsample_time_by='auto', this determines the temporal downsampling factor by setting a minimum event tau after downsampling. Default:5. -
reestimate_S_if_downsampled: When set totrue, images are re-estimated from full movie at the end. Whenfalse, images are upsampled by interpolation.reestimate_S_if_downsampled=trueis not recommended as precise shape of cell images are typically not essential, and re-estimation from full movie is costly. -
verbose: Log is emitted from the console output when set to1, set to0to suppress output. When set to2, EXTRACT provides a detailed summary during the signal extraction process. Default:2. -
crop_cicrcular: For microendoscopic movies, set it totruefor automatically cropping out the region outside the circular imaging region. Default:false. -
dendrite_aware: Boolean flag, set it totrueif dendrites exist in the movie & are desired in the output. Default:false. -
adaptive_kappa: Iftrue, then during cell finding, the robust esimation loss will adaptively set its robustness parameter. Default:false. -
smoothing_ratio_x2y: If the movie contains mainly objects that are elongated in one dimension (e.g. dendrites), this parameter is useful for more smoothing in either x or y dimension. Default:1. -
compact_output: If set totrue, then the output will not include bad components that were found but then eliminated. This usually reduces the memory used by the output struct substantially. Default:true. -
use_sparse_arrays: If set totrue, then the output cell images will be saved as sparse arrays. Default:true. -
temporal_denoising: Boolean flag that determines whether to apply temporal wavelet denoising. This functionality is experimental; expect it to increase runtime considerably if the input movie has >10K frames and hase larger field of view than 250x250 pixels. Default:false. -
remove_background. Boolean flag that determines whether to subtract the (spatially) background (largest spatiotemporal mode of the movie matrix). Default:true. -
cellfind_max_steps: Maximum number of cell candidate initialization during cell finding step. Default:1000. -
cellfind_kappa_std_ratio: Kappa will be set to this times the noise std for the component-wise EXTRACT during initialization. Default:1. -
cellfind_filter_type: Type of the spatial smoothing filter used for cell finding. Options:'butter'(IIR butterworth filter),'gauss'(FIR filter with a gaussian kernel),'wiener'(wiener filter),'movavg'(moving average in space),'none'(no filtering). Default:'butter'. -
spatial_highpass_cutoff,spatial_lowpass_cutoff: These cutoffs determine the strength of butterworth spatial filtering of the movie (higher values = more lenient filtering), and are relative to the average cell radius. Defaults:5&2. -
init_with_gaussian: If true, then during cell finding, each cell is initialized with a gaussian shape prior to robust estimation. If false, then initialization is done with a correlation image (preferred for movies with dendrites). Default:false. -
cellfind_numpix_threshold: During cell finding, objects with an area <cellfind_numpix_thresholdare discarded. Default:9. -
S_init: Optionally, provide cell images inconfig.S_initas a 2-D matrix (with the size of the first dimension equal to movie height x movie width), and the algorithm will use these as the initial set of cells, skipping its native initialization. Default: empty array. -
smooth_T: If set totrue, calculated traces are smoothed using median filtering. Default :false. -
smooth_S: If set totrue, calculated images are smoothed using a 2-D gaussian filter. Default :true. -
max_iter: Maximum number of alternating estimation iterations. Default :6. -
plot_loss: When set totrue, empirical risk is plotted against iterations during alternating estimation. Default:false. -
l1_penalty_factor: A numeric in range[0, 1]which determines the strength of l1 regularization penalty to be applied when estimating the temporal components. The penalty is applied only to cells that overlap in space and whose temoral components are correlated. Use larger values if spurious cells are observed in the vicinity of high SNR cells. Default:0. -
max_iter_S,max_iter_T: Maximum number of iterations forSandTestimation steps. Default:100and100. -
TOL_sub: If the 1-step relative change in the objective within eachTandSoptimization is less than this, the respective optimization is terminated. Default:1e-6. -
kappa_std_ratio. Kappa will be set to this times the noise std during the cell refinement process. Lower values introduce more robustness at the expense of an underestimation bias inSandT(especially in the low SNR regime). Default :1. -
TOL_main: If the relative change in the main objective function between 2 consecutive alternating minimization steps is less than this, cell extraction is terminated. Default:1e-6. -
medfilt_outlier_pixels: Flag that determines whether outlier pixels in the movie should be replaced with their neighborhood median. Default:false. -
remove_duplicate_cells: For movies processed in multiple partitions, this flag controls duplicate removal in the overlap regions. Default:true. -
T_dup_corr_thresh,S_dup_corr_thresh: Through alternating estimation, cells that have higher trace correlation thanT_dup_corr_threshand higher image correlation thanS_dup_corr_threshare eliminated. Defaults:0.95&0.95. -
temporal_corrupt_thresh,spatial_corrupt_thresh: Spatial & temporal corruption indices are calculated at each step of the alternating minimization routine. Images / traces that have an index higher than these are eliminated. Defaults :0.7&0.7. -
T_min_snr: Cells with lower SNR value thanT_min_snrwill be eliminated. Default:10.
We suggest to check get_defaults.m for further info on how to set these parameters.
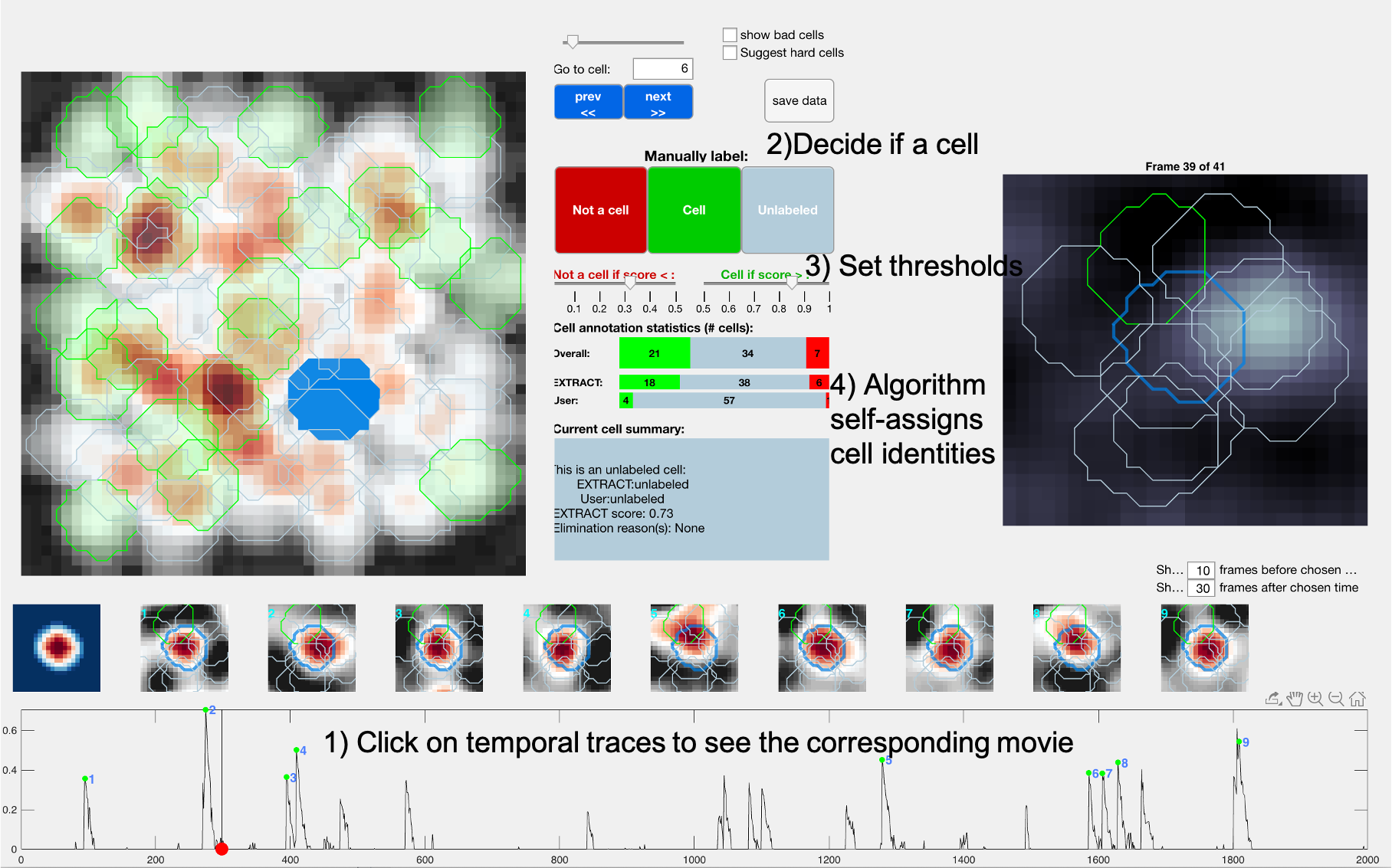
EXTRACT has an internally built-in cell-check algorithm, which employs semi-supervised machine learning methods to aid the user with the cell checking process. The corresponding file is cell_check.m, which takes in two inputs: M and output. M is the movie, whereas output is the output generated by EXTRACT that contains cell maps and temporal traces. It has the following properties:
- The user can observe the cell maps during spiking times or click on the temporal trace map to watch the raw movie during that time window.
- The user can decide whether a candidate is indeed a cell or not.
- The algorithm performs some computations in the background to assist the user, where the user can decide on some acceptance and rejection thresholds.
- Once a small portion of cell candidates are checked, the algorithm provides a guess for all the cell candidates. Thus, one does not need to check all the cells.
The figure below explains the process in 4 steps. In this example, the user had checked only 5 cell candidates and EXTRACT identified 18 cells and 6 non-cells. We note that this feature is still experimental and we are constantly working to improve it. We are also providing an external cell checker in case cell_check fails, which usually happens when the number of EXTRACT partitions is larger than 1.
There is also an external cell checker, which is part of the CIAtah pipeline. After downloading and including the pipeline in the MATLAB path, one can use the following code to run the EXTRACT output on the cell checker of CIAtah pipeline:
% EXTRACT output is stored in a structure called "output"
% Some configurations for the external cell checker
iopts.inputMovie = M; % movie associated with traces
iopts.valid = 'neutralStart'; % all choices start out gray or neutral to not bias user
iopts.cropSizeLength = 20; % region, in px, around a signal source for transient cut movies (subplot 2)
iopts.cropSize = 20; % see above
iopts.medianFilterTrace = 0; % whether to subtract a rolling median from trace
iopts.subtractMean = 0; % whether to subtract the trace mean
iopts.backgroundGood = [208,229,180]/255;
iopts.backgroundBad = [244,166,166]/255;
iopts.backgroundNeutral = repmat(230,[1 3])/255;
% Run the external cell checker
[inputImagesSorted, inputSignalsSorted, choices] = ciapkg.classification.signalSorter(output.spatial_weights, output.temporal_weights', 'options',iopts);EXTRACT requires, at least, the following toolboxes. With future releases, there may be need for further toolboxes.
- Bioinformatics Toolbox
- Econometrics Toolbox
- Image Processing Toolbox
- Parallel Computing Toolbox
- Signal Processing Toolbox
- Statistics and Machine Learning Toolbox
- Wavelet Toolbox
EXTRACT is mainly written by Hakan Inan in collaboration with many researchers in Schnitzerlab. The database is maintained by the current members of Schnitzerlab. If you have any questions or comments, after checking already existing issues and the Frequently asked questions section, please open an issue or contact via email extractneurons@gmail.com.
Copyright (C) 2020 Hakan Inan
Licensed under the MIT license:
http://www.opensource.org/licenses/mit-license.php
Permission is hereby granted, free of charge, to any person obtaining a copy of this software and associated documentation files (the "Software"), to deal in the Software without restriction, including without limitation the rights to use, copy, modify, merge, publish, distribute, sublicense, and/or sell copies of the Software, and to permit persons to whom the Software is furnished to do so, subject to the following conditions:
The above copyright notice and this permission notice shall be included in all copies or substantial portions of the Software.
THE SOFTWARE IS PROVIDED "AS IS", WITHOUT WARRANTY OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO THE WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND NONINFRINGEMENT. IN NO EVENT SHALL THE AUTHORS OR COPYRIGHT HOLDERS BE LIABLE FOR ANY CLAIM, DAMAGES OR OTHER LIABILITY, WHETHER IN AN ACTION OF CONTRACT, TORT OR OTHERWISE, ARISING FROM, OUT OF OR IN CONNECTION WITH THE SOFTWARE OR THE USE OR OTHER DEALINGS IN THE SOFTWARE.
This version of EXTRACT is written in Matlab and will be improved over the years in terms of both speed and memory consumption. Furthermore, we plan to release a Python version in the near future.
EXTRACT is regularly maintained by the current members of the Schnitzerlab. Unless this answer changes, this will be the case.
EXTRACT can be used for cell extraction from both one-photon and two-photon calcium imaging movies.
EXTRACT requires that the user had already performed motion correction and there is an option in the state-of-the-art motion correction algorithms to save the output as an hdf5 file (See for example: NoRMCorre). We do not advise running EXTRACT without motion correcting first.
The current suggested workflow for running EXTRACT is the following: One performs the motion correction on the imaging file (which can be saved in many different ways, .avi, .tiff and/or .tif) and saves the motion corrected output as hdf5. We find that hdf5 works fastest and most convenient for EXTRACT pipeline. That being said, we intend to add support for various input file types to the EXTRACT pipeline in the future.
I am receiving an error when running the EXTRACT algorithm or the algorithm finds no cells. What are the most common reasons?
EXTRACT has been used by many members of Schnitzerlab in the last few years for various types of movies. If an error occurs, it is usually due to one of the following reasons:
- The raw movie is not properly motion corrected.
- The raw movie includes artifacts and/or inf values. Large artifacts existing in even few frames can cause the pre-processing step to fail or result in zero found cells. While EXTRACT performs well in low SNR movies, large movie artifacts tend to throw off both motion correction and cell extraction algorithms.
-
avg_cell_radiusis too low or too high. - The input movie is low SNR and
cellfind_min_snris set too high. - If you are receiving a memory error, most likely the RAM is not sufficient for the process. In this case, we advise to increase the number of space partitions.
We tend to visually inspect the motion corrected movies for any artifacts that might exist in the raw movie or happen during the motion correction step. Whatever the error may be, it usually originates from sources outside of EXTRACT.
EXTRACT uses a set of thresholds decreasing the time that the user needs to spend on cell checking. If for a particular movie, the false-negative count is high, one can decrease the value of cellfind_min_snr and/or T_min_SNR. It may also be the case that cellfind_max_steps is set too low.
Following the logic of the previous question, if for a particular movie, the false-positive count is high, one can increase the value of cellfind_min_snr and/or T_min_SNR. This is rarely the case for EXTRACT, as many thresholding metrics ensures high precision values for typical movies.
During the preprocessing module, EXTRACT performs a stationary background removal step. In some movies, during this step, the time-dependent background removal might lead to negative going spikes. EXTRACT has a built-in assumption to threshold any negative activity, thus the negative going spikes do not show-up during the usual EXTRACTion process, but only during the (optional) final robust regression of raw traces. In these cases, please set remove_background=0 in the configuration file and run EXTRACT. Note that if this is done, it is important that one has a movie with a fairly stationary background throughout time points.
Our general suggestion is to keep remove_background=1 in all cases, use non-negative traces for data analysis and use raw traces (that might contain some negative spikes here and there) for noise estimation. Once noise is estimated from the raw traces, we suggest to perform thresholding to the traces (max(0,traces)), which turns them into non-negative traces. This is consistent with EXTRACT's inherent assumption that signal is non-negative.
While cellfind_kappa_std_ratio sets the kappa value for the cell finding process, kappa_std_ratio sets the kappa value for the cell refinement process.
In some cases, it may be beneficial to monitor the algorithm while it is running. In such cases, we suggest keeping config.verbose=2, which provides more detailed information of how many cells are found initially and how many are discarded during cell refinement processes. This way, one can monitor whether the thresholds are set too high or too low.
Citation pour cette source
Inan, Hakan, et al. Fast and Statistically Robust Cell Extraction from Large-Scale Neural Calcium Imaging Datasets. Cold Spring Harbor Laboratory, Mar. 2021, doi:10.1101/2021.03.24.436279.
Compatibilité avec les versions de MATLAB
Plateformes compatibles
Windows macOS LinuxCatégories
Tags
Community Treasure Hunt
Find the treasures in MATLAB Central and discover how the community can help you!
Start Hunting!Découvrir Live Editor
Créez des scripts avec du code, des résultats et du texte formaté dans un même document exécutable.
EXTRACT
EXTRACT/main_functions/debug_utils
EXTRACT/main_functions/debug_utils/brewermap
EXTRACT/main_functions/helper_functions
EXTRACT/main_functions/solvers/base solvers for multiple cells
EXTRACT/main_functions/solvers/cell finding solvers
EXTRACT/main_functions/solvers/cell refinement + frr solvers
EXTRACT/modules
EXTRACT/summary functions
External algorithms
External algorithms/parfor_progress
External algorithms/progressbar
Learning materials/Lecture Tutorials/Tutorial 1 - Starting code
Learning materials/Lecture Tutorials/Tutorial 2 - Understanding hyperparameters
Learning materials/Lecture Tutorials/Tutorial 3 - Hyperparameter optimization
Learning materials/Lecture Tutorials/Tutorial 4 - Low SNR movies
Learning materials/NWB Tutorials
Template scripts
Learning materials/Live code examples
Les versions qui utilisent la branche GitHub par défaut ne peuvent pas être téléchargées
| Version | Publié le | Notes de version | |
|---|---|---|---|
| 1.0.0 | See Github page |
|
|
| 0.7.3 | Proper citation added |
|
|
| 0.7.2 |
|